Contact angle
A "contact angle" is an important concept in surface science and physics that helps us understand how liquids interact with solid surfaces. It measures the angle at which a liquid droplet sits on a surface. This angle tells us whether the liquid spreads out or forms into droplets on the surface, and it depends on the forces between the liquid and the surface.
Contact angle measurements are used in many different fields like materials science, chemistry, and engineering. They give us useful information about how liquids behave on different surfaces and how easily they wet the surface. This knowledge has practical applications in various areas like making better adhesives, creating coatings, and developing surfaces that are easy to keep clean and repel water.
Young equation
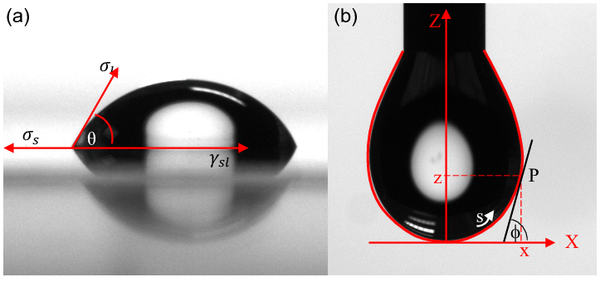
The contact angle (θ) refers to the tangent line to the angle formed at the point of contact where the liquid touches the solid.
The contact angle is shown in Young's equation in the illustration below.


Measurement methods
Sessile Drop Contact Angle (a): This angle, denoted as θ, is the angle formed between the solid surface and the tangent line drawn at the edge of a liquid drop resting on that surface. If the liquid drop spreads out and wets the surface effectively, the contact angle is small. Conversely, if the drop forms a bead and does not wet the surface, the contact angle is large.
Pendant Drop Contact Angle (b): This angle is determined in a manner similar to the Sessile Drop method but is applicable when a liquid drop is suspended or "hanging" from a surface. Like the Sessile Drop method, it provides information about the wetting characteristics of the surface.
Advancing and Receding Contact Angles: These angles describe how the contact angle changes as a liquid drop either spreads or recedes on a surface. The advancing angle represents the maximum angle achieved as the drop spreads, while the receding angle corresponds to the minimum angle observed as the drop recedes from the surface. These angles offer insights into dynamic wetting behavior.
Sessile Drop
The Sessile Drop method is a way to measure how well a liquid wets a solid surface. By analyzing the angle formed between a liquid droplet and the surface, we can understand how the liquid interacts with the surface. This method is often used to investigate contact angles and is considered a fundamental technique in this field.
Water is usually the liquid of choice for these measurements, and the angle formed by the water droplet can tell us whether the surface is hydrophobic or hydrophilic. Hydrophilicity is particularly important when it comes to coatings, as it determines how well the surface can be wetted. The Sessile Drop method is often used as a quality control tool before applying coatings to make sure the surface has the necessary properties for optimal performance.
Droplet Deposition
A small drop of liquid is delicately placed onto a flat, solid surface. Typically, the drop adheres to the surface, taking on a "flat" shape.
Image and Analysis
A camera or imaging system captures an image of the droplet on the surface, which is subsequently used for calculating and analysing the droplet's shape.
Measurement of Contact Angle
The contact angle is determined by measuring the angle formed between the tangent line at the point where the droplet's edge intersects the solid surface.
Surface Characterization
This process aids scientists and engineers in comprehending how a liquid behaves on a solid surface—whether it spreads out or forms beads. A small contact angle indicates effective wetting, where the liquid tends to spread and adhere to the surface (hydrophilic). Conversely, a large contact angle signifies poor wetting, resulting in the liquid adopting a more spherical shape, indicating limited interaction with the surface (hydrophobic).
Determination of Surface Energy
Contact angles measured using various liquids can be utilized to compute the surface energy of the solid material. This is crucial for understanding material adhesion and compatibility across diverse applications.
Quality Control
Industries such as manufacturing, coating, and electronics employ the sessile drop method for quality control, ensuring that surfaces exhibit the desired wetting properties.
Materials Development
Researchers utilize this method for designing and modifying materials tailored to specific applications, including the development of superhydrophobic or superoleophobic coatings.


Pendant Drop
The Pendant Drop contact angle is another method used to measure contact angles, similar to the sessile drop method. However, in the pendant drop method, the liquid drop is suspended from a solid surface instead of being in direct contact with it. This technique is useful for examining the wetting properties of a solid surface and understanding how different liquids behave.
The Pendant Drop method is a valuable tool that complements the sessile drop method. It provides additional insights that are applicable across various industries, including coatings, adhesives, pharmaceuticals, and petrochemicals. The information obtained through the pendant drop method helps researchers and engineers make informed decisions and improvements in these industries.
Drop formation
A small drop of a liquid is dispensed from a fine needle or tube and it hangs from the end of the needle, forming a pendant drop.
Camera and imaging
A camera is used to capture an image of the droplet, including its shape and curvature.
Measurement of contact angle
The contact angle is determined by analyzing the shape of the drop. The angle formed between the tangent line at the bottom of the drop and the surface of the drop itself is the contact angle.
Surface characterization
The method provides insight into the wetting behavior of a liquid on a solid surface and helps to understand how effectively a surface repels or interacts with different liquids.
Measurement of surface tension
By examining the shape and curvature of the drop, one can also calculate the surface tension of the liquid.
Interface properties
The method can be used to investigate the interface properties between different phases (e.g. solid-liquid, liquid-gas or liquid-liquid).
Determination of surface energy
Just like the sessile drop method, the pendant drop method can be used to determine the surface free energy of solids, especially by measuring the contact angles of different probe liquids.
Advancing & Receding
Advancing and Receding contact angles are two additional angles that go beyond the static or equilibrium contact angle measured in the sessile or pendant drop methods. They provide insights into how a liquid interacts with a solid surface as it undergoes the processes of wetting or drying. These dynamic contact angles help us understand how liquids behave on surfaces, especially when external forces or changes in liquid volume come into play.
Understanding both the advancing and receding contact angles is crucial for gaining insights into the dynamic wetting properties of surfaces. This knowledge is valuable in various fields, including materials science, surface chemistry, and manufacturing processes. These measurements are often used to optimize surface designs, coatings, and materials for specific applications where precise control over moisture behaviour is necessary.
Advancing contact angle
Definition: The advancing contact angle is the maximum contact angle observed. That is, a liquid drop that spreads over a solid surface.
Measurement: This angle is usually measured while the liquid is "advancing", meaning it spreads or advances on the surface. It occurs when the droplet size increases or an external force (such as a pump or pressure) is applied to increase the droplet's volume.
Importance: The advancing contact angle gives insight into how easily a liquid can wet the surface. A smaller advancing contact angle indicates that the liquid spreads easily, suggesting good wetting and surface affinity. This measurement is often relevant in applications such as coatings and adhesion processes.
Decreasing contact angle
Definition: The receding contact angle is the smallest contact angle observed when a droplet begins to recede or withdraw from a solid surface.
Measurement: This angle is usually measured when the liquid recedes, meaning it recedes or dehumidifies from the surface. It occurs when the droplet size decreases or when an external force is applied to reduce the droplet's volume.
Importance: The decreasing contact angle helps determine the resistance of a liquid to leave the surface. A less decreasing contact angle suggests that the liquid decreases more easily and does not adhere strongly to the surface. This is important for understanding surface cleaning, "non-stick" coating and prevention of unwanted adhesion.
The difference between the advancing and receding contact angle, known as the contact angle hysteresis, reflects the stability of the liquid-solid interface. A large hysteresis indicates that the liquid adheres well to the surface, while a small hysteresis implies a more stable and reversible wetting behavior.



Hydrophilic, hydrophobic and superhydrophobic
The contact angle is usually measured between the tangent line at the point of contact on the solid surface and the two sides of the liquid droplet. It can be categorized into three primary regimes:
Acute angle (θ <90 degrees): In this case, the liquid wets the surface, which means it spreads out and sticks to the surface. A contact angle less than 90 degrees indicates hydrophilicity, where the liquid is attracted to the surface and tends to spread.
Right angle (θ = 90 degrees): This is referred to as the "Young-Laplace contact angle." When the contact angle is exactly 90 degrees, it means that the liquid neither moistens nor is repelled by the surface. It maintains a spherical shape.
Obtuse angle (θ> 90 degrees): An obtuse contact angle indicates that the liquid is repelled by the surface, forming a more spherical shape. A contact angle greater than 90 degrees indicates hydrophobicity, where liquid beads adhere minimally to the surface.
